|
The Basics - Polymer Definition &
Properties
If you're after basic information on plastic materials, this is
the place to find it. Here you'll learn the definition and
properties of polymers, the building blocks of plastics.
Plastics are polymers. What is a polymer? The most simple
definition of a polymer is something made of many units. Think of
a polymer as a chain. Each link of the chain is the "-mer" or
basic unit that is usually made of carbon, hydrogen, oxygen
and/or silicon. To make the chain, many links or "-mers" are
hooked or polymerized together. Polymerization can be
demonstrated by linking countless strips of construction paper
together to make paper garlands or hooking together hundreds of
paper clips to form chains, or by a string of beads.
Plastic materials display properties that are unique when
compared to other materials and have contributed greatly to
quality of our everyday life. Plastics, properly applied, will
perform functions at a cost that other materials cannot match.
Many natural plastics exist, such as shellac, rubber, asphalt,
and
cellulose ; however, it is man's ability to synthetically
create a broad range of materials demonstrating various useful
properties that have so enhanced our lives. Plastics are used in
our clothing, housing, automobiles, aircraft, packaging,
electronics, signs, recreation items, and medical implants to
name but a few of their many applications.
The synthetic plastic industry started in
1909 with the development of a phenol formaldehyde plastic
(Bakelite) by Dr. L. H. Bakeland. The phenolic materials are,
even today, important engineering plastics. The development of
additional materials continued and the industry really began to
blossom in the late 1930's. The chemistry for nylons, urethanes,
and fluorocarbon plastics were developed; the production of
cellulose acetate, melamine, and styrene molding compounds began;
and production of commercial equipment to perform the molding and
vacuum forming processes began.
Acrylic sheet was widely used in aircraft
windows and canopies during World War II. A transparent polyester
resin (CR-39), vinylidene chloride film (Saran), polyethylene,
and silicone resins were also developed. The first polyethylene
bottles and cellulose acetate toothpaste tubes were manufactured
during this time period.
The post war era saw the production of vinyl
resins started, the use of vinyl films, molded automotive acrylic
taillights and back-lighted signs introduced, and the first
etched circuit boards developed. The injection molding process
entered commercial production. Due to the newness of the
materials, the properties and behavior of the plastic materials
were not well understood. Many products were introduced that
failed, creating a negative impression about plastics in the
public's mind.
Chemists continued the development of
materials, such as ABS, acetals, polyvinyl fluoride, ionomers,
and polycarbonate. The injection molding, thermoforming,
extrusion, transfermolding, and casting processes were all
improved. This allowed the industry to provide an even greater
number of cost-effective products suitable for many, more
demanding engineering applications.
In the
early days...
Bakelite (phenolic resin) is most often actually a
product called Catalin. Both, along with Plaskon, are
formaldehyde based plastics. Allow me to expand (with liberal
borrowing from Dr. Stephen Z. Fadem - a true expert).
Around the turn of the century, the Belgian
born scientist Dr. Leo Baekeland, working as an independent
chemist, came upon the compound quite by accident. He sold his
rights to Velox to Eastman Kodak for three quarters of a million
dollars and started developing a less flammable bowling alley
floor shellac; bowling was becoming the latest rage in New York
City. Dr. Baekeland soon realized that a resin that was both
insoluble and infusible could have a much wider appeal when used
as a molding compound. He obtained a patent and started the
Bakelite Corporation around 1910.
Phenolic resin could be produced in a
multitude of colors, commonly yellow, brown, butterscotch, green
and red. Omitting the pigment could produce a transparent or
translucent effect. The resin could be molded or cast, depending
on variations in the formula. For molding, the formula was cooked
until resinous, spread out in thin sheets to harden, then ground
to a fine consistency. At this point, powdered fillers and
pigment were added, to enable the resin to be molded and to add
color. This mixture was then put through hot rollers which
created large sheets of colored, hardened resin. These sheets
were then ground into a very fine powder which was molded under
high heat and pressure into the final product form. As a molded
material the resin's drawback was the limited range of colors
which could be created. For casting, the formula was modified
slightly, enabling the resin to be poured into lead molds and
then cured in ovens until it polymerized into a hard substance.
The liquid resin could be tinted to any color or "marbleized" by
mixing two colors together.
For the first ten years or so after its
introduction, the resin was used primarily to make electrical and
automobile insulators and heavy industrial products. Eventually,
uses for the resin spread into the consumer market. Castings were
made in the shape of cylinders or blocks, and then sold to
novelty and jewelry makers. Industrial designers began
experimenting with the new material. Fine craftsmen sculpted the
molded products on fast wheels with razor-like tools to carve out
designs that the world has not seen since; after World War II,
most companies switched to creating designs through the use of
patterned molds, instead of hand-carving. Bakelite replaced
flammable celluloid, previously the most popular synthetic
material for molded items, as a major substance for jewelry
production.
The process to the collector of today may
not be significant, as Bakelite is now treasured for its unique,
unreproducible beauty. A deeply carved half inch bangle bracelet
may sell for $225.00, and a two and one half inch bangle may
command $900.00. Bakelite often acquires a patina within a few
months to a few years of its date of production, and
metamorphisizes into a completely different appearing color. The
red, white and blue Bakelite designs of yesterday have mellowed
into lovely yellows, reds and blacks, enhancing further the value
of those rare pieces which have continued to maintain their
original color and luster.
Bakelite's many uses allowed it to become a
standard item in the family home of the 1930s and 1940s. It was
frequently found in the kitchen, in the form of flatware handles,
rabbit or chicken napkin holders, salt and pepper shakers, or
serving trays. During the Depression Bakelite sold more than any
other commercial product, and was loved by the public for its
brilliant and cheerful colors and its affordability.
When the Bakelite patent expired in 1927, it
was acquired by the Catalin Corporation that same year. They
began mass production under the name "Catalin," using the cast
resin formula which enabled Catalin to add 15 new colors to the
original five produced by the Bakelite Corporation, which used
the limited color range molded formula, as well as the now-famous
marbleized effect. One of their most notable products was the
Fada bullet radio. The Catalin Corporation was responsible for
nearly 70% of all phenolic resins that exist today.
Bakelite-Catalin was sold mostly by Saks
Fifth Avenue, B. Altman and Bonwit Teller, but was also on the
shelves of F.W. Woolworth and Sears. To the wealthy socialites,
whose husbands had fallen on tough times during the Depression,
with Tiffany diamonds and Cartier jewelry now well beyond their
means, the vibrantly colorful carved jewelry adorned with
rhinestones became de riguer for cocktail parties and formal
dinners. Yet, Catalin and Bakelite were within everyone's reach
with Depression prices ranging from twenty cents to three
dollars. Diana Vreeland, editor of Vogue, often spoke of the
versatility of Bakelite, as did Elsa Schiaparelli, who was
constantly contracting with the Bakelite and Catalin Corporations
for exclusive buttons for her dress designs.
But in 1942 Bakelite and Catalin suspended
sales of their colorful cylinders to costume jewelry
manufacturers in order to concentrate on the wartime needs of a
nation which had totally shifted its focus. Defense phones and
aviator goggles, as well as thousands of other Bakelite products,
found their way to armed forces around the world. The scheme
shifted from the 200 vibrant colors which brightened the dark
days of the Depression to basic black, the no-nonsense symbol of
a nation at war. By the end of the war, new technology had given
birth to injection-molded plastics, and most manufacturers
switched to less labor-intensive and more practical means of
developing products. The next generation of plastics had been
born - Acrylic, fiberglass, and vinyl - and they were molded into
products commonplace in our everyday lives today.
Occasionally plastics are still improperly
used and draw negative comments. The thousands of successful
applications that contribute to the quality of our life are
seldom noticed and are taken for granted. Remember, MATERIALS
DON'T FAIL, DESIGNS DO.
The number of variations or formulations
possible by combining the many chemical elements is virtually
endless. This variety also makes the job of selecting the best
material for a given application a challenge. The plastics
industry provides a dynamic and exciting opportunity.
Plastics encompass a large and varied group
of materials consisting of different combinations or formulations
of carbon, oxygen, hydrogen, nitrogen and other organic and
inorganic elements. Most plastics are a solid in finished form;
however, at some stage of their existence, they are a liquid and
may be formed into various shapes. The forming is usually done
through the application, either singly or together, of heat and
pressure. There are over fifty different, unique families of
plastics in commercial use today and each family may have dozens
of variations.
MONOMERS: A, B, C
Examples of monomers are ethylene, styrene, vinyl chloride
and propylene.
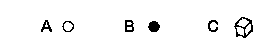
Figure 1a
HOMOPOLYMERS: A-A-A-A-A-A-A-A-A-
(Polymers constructed from a single material)
Examples of polymers built this way are polyethylene, and some
acetals.
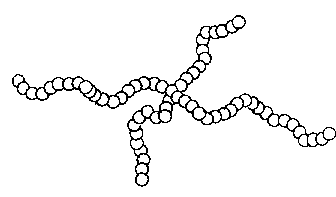
Figure 1b
COPOLYMERS:
(Polymers constructed from two different materials)
ALTERNATING TYPES: A-B-A-B-A-B-A-B-A-B-
Some examples of alternating copolymers are ethylene-acrylic and
ethylene-ethyl acrylate.
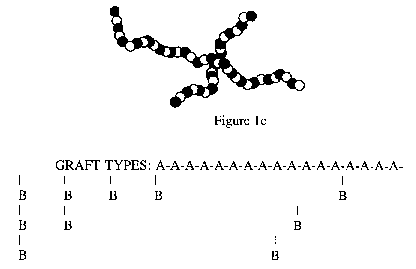
Some examples of grafted copolymers are
styrene-butadiene, styrene-acrylonitrile, and some acetals.
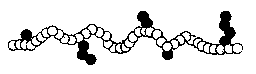
Figure 1d
TERPOLYMERS: A-B-C-A-B-C-A-B-C-
(Polymers constructed from three different materials)
An example of a terpolymer is acrylonitrile-butadiene-styrene
(ABS).
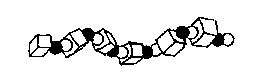
Figure 1e
The two monomers in a copolymer are combined
during the CHEMICAL REACTION of polymerization. Materials called
"ALLOYS" are manufactured by the SIMPLE MIXING of two or more
POLYMERS with a resulting blending of properties which are often
better than either individual material. There is no chemical
reaction in this process. Some examples of "alloys" are
Polyphenylene Oxide/High Impact Styrene, Polycarbonate/ABS, and
ABS/PVC.
MOLECULAR WEIGHT
It is important for the chemist to know how
long the polymer chains are in a material. Changing the length of
the chains in a thermoplastic material will change its final
properties and how easily it can be shaped when it is melted.
The "REPEATING UNIT" or molecular group in
the homopolymer (Figure 1) is A-, the group of molecules in the
copolymer A-B-, and in the terpolymer A-B-C-. The number of
repeating units in the polymer chain is called the "DEGREE OF
POLYMERIZATION." If the repeating unit has a molecular weight
(the combined weight of all of the molecules in the repeating
unit) of 60 and the chain or polymer has 1000 repeating units,
then the polymer has a "MOLECULAR WEIGHT" of 60 x 1000 = 60,000.
The molecular weight is a way of measuring how long the polymer
chains are in a given material.
The molecular weight of plastics is usually
between 10,000 and 1,000,000. It becomes increasingly difficult
to form or mold the plastic with the application of heat and
pressure as the molecular weight increases. A molecular weight of
about 200,000 is about the maximum for a polymer to still permit
reasonable processability. Some higher molecular weight
materials, like Ultra High Molecular Weight Polyethylene (UHMWPE)
which has a molecular weight from 3,000,000 to 6,000,000, can be
cast using processes specifically designed to shape it.
Molecular Arrangement of Polymers
Think of how spaghetti noodles look on a plate. This is similar
to how polymers can be arranged if they lack a specific for or
are amorphous. Controlling and quenching the polymerization
process can result in amorphous organization. An amorphous
arrangement of molecules has no long-range order or form in which
the polymer chains arrange themselves. Amorphous polymers are
generally transparent. This is an important characteristic for
many applications such as food wrap, plastic windows, headlights
and contact lenses.
Obviously not all polymers are transparent. The polymer chains in
objects that are translucent and opaque are in a crystalline
arrangement. By definition a crystalline arrangement has atoms,
ions, or in this case, molecules in a distinct pattern. You
generally think of crystalline structures in salt and gemstones,
but not in plastics. Just as quenching can produce amorphous
arrangements, processing can control the degree of crystallinity.
The higher the degree of crystallinity, the less light can pass
through the polymer. Therefore, the degree of translucence or
opaqueness of the polymer is directly affected by its
crystallinity.
Scientists and engineers are always producing better materials by
manipulating the molecular structure that affects the final
polymer produced. Manufacturers and processors introduce various
fillers, reinforcements and additives into the base polymers,
expanding product possibilities.
The Structure of Polymers
Many common classes of polymers are composed of hydrocarbons.
These polymers are specifically made of small units bonded into
long chains. Carbon makes up the backbone of the molecule and
hydrogen atoms are bonded along the backbone.
There are polymers that contain only carbon and hydrogen.
Polypropylene, polybutylene, polystyrene and polymethylpentene
are examples of these.
Even though the basic makeup of many polymers is carbon and
hydrogen, other elements can also be involved. Oxygen, chorine,
fluorine, nitrogen, silicon, phosphorous and sulfur are other
elements that are found in the molecular makeup of polymers.
Polyvinyl chloride (PVC) contains chlorine. Nylon contains
nitrogen. Teflon contains fluorine. Polyester and polycarbonates
contain oxygen. There are also some polymers that, instead of
having a carbon backbone, have a silicon or phosphorous backbone.
These are considered inorganic polymers. One of the most famous
silicon-based polymers is Silly Putty.
The Structure of Polymers More
8
Thermal Properties of Polymers
More
8
|
Kinds
of Polymers |
|
|
- Aramids
- Polycarbonate
- Polyester
- PMMA
- Polyurethane
- Polystyrene
- SBS Rubber
- Silicone
- PTFE
- Polymer Topics
- Tough and Hard
- Bend and Stretch
- Natural Polymers
- Synthetic Polymers(made by people)
- Information Booth:
|
| 

 Knowledge Base
Knowledge Base







 Knowledge Base
Knowledge Base

















 Knowledge Base
Knowledge Base


